Laser scanning microscopy
Background of the project
Every year, about 500,000 people in Germany are diagnosed with cancer. Diagnostics and treatment are constantly developing. Currently, surgery, chemotherapy and radiotherapy are used as standard treatment options. Immunotherapy using therapeutic antibodies and genetically modified, endogenous immune cells (CAR-T cells) has recently moved into focus.
The distinction between healthy tissue and tumor tissue is often not easy. The aim of tumor surgery is to maintain tumor-free resection margins while at the same time increasing the preservation of surrounding healthy tissue. Possible problems are the following:
- differentiation between healthy and tumorous tissue is subjectively assessed by the surgeon
- 3-dimensional display of the tumor border is not possible
- histology is usually performed postoperatively
- Quick tissue sections during surgery are possible, but
- tissue sections only provide suggestions
- frozen sections of fatty tissue are technically difficult
- minimum sample sizes required
- some tissues cannot be processed at all (e.g. bone)
- intra-operative application depending on the transport route are
timeconsuming
Project objective
Development of an easy-to-use optical method, that:
- can distinguish intraoperatively healthy tissue structures from tumor tissue
- ensures the complete preservation of cranial nerves and arteries during e.g. neurosurgical interventions
- enables visualization of specific staining of tumor tissue
- visualizes reliable results within an acceptable time frame
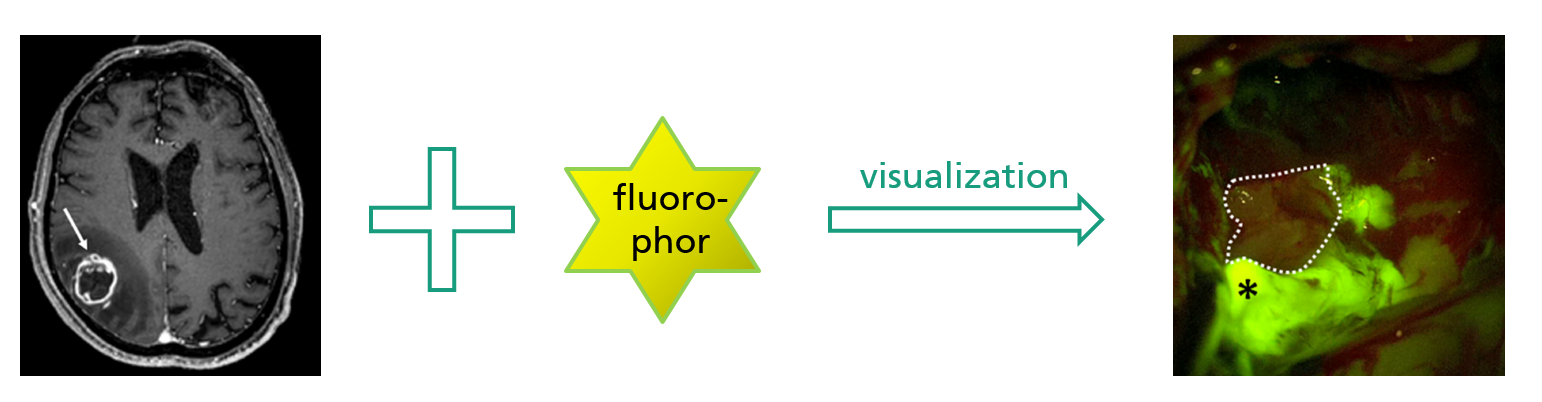
Project idea
Acquisition of optical sectional images using confocal MEMS-based laser fluorescence microscopy in vivo.
Project implementation
Microscope development
Within the project, a laboratory demonstrator of a miniaturized confocal laser scanning microscope is to be developed. The advantages of the development should be the use of a MEMS device (Micro-Electro-Mechanical System) developed at the Fraunhofer IPMS and thus the miniaturization and the cheaper production compared to competing technologies. The proof-of-concept for a robust MEMS-based fluorescence laser scanning microscope has already been provided2,3 and is protected by patent (DE102013222349B3 of Febr 15, 2015).
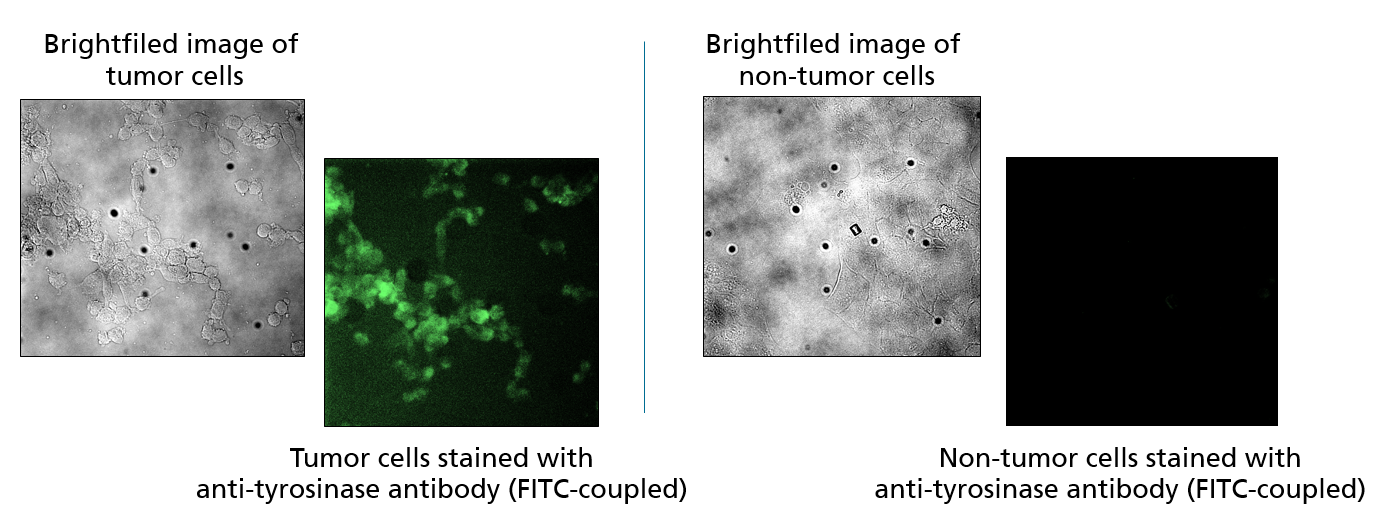
Differentiation between tumor and healthy tissue
The further development of the demonstrator should allow its application in surgically accessible tumors (e.g. brain or skin tumors). To this end, a method for the specific staining of tumor cells using fluorescence-labeled antibodies at the cell culture level was established at Fraunhofer IZI. In a next step, patient material will be used and the specific staining of tumor cells will be visualized on 2D sections of patient material. Subsequently, the possibility of using the demonstrator on 3D tissue samples from patients outside the body (ex vivo) will be investigated in order to work out an intraoperative application on the patient (in vivo).
Research Fraunhofer IZI, fluorescent dyes - approved for use in humans:
| dye | wave length | notes |
| fluorescein | excitation maximum: 485 nm (pH 9) emission maximum: 514 nm (pH 9) |
|
| indocyanine green (ICG) | excitation maximum: 600 – 900nm emission maximum: 750 – 950 nm |
|
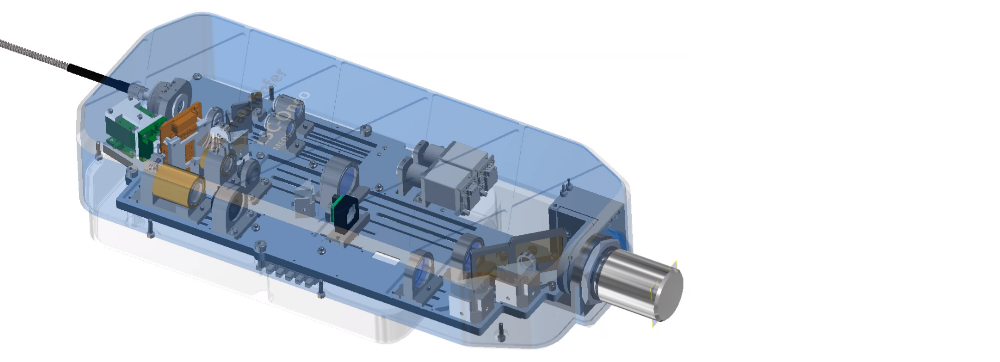
Project result
The realized LSC demonstrator can be operated via a specially programmed user interface. It takes fluorescence images at two laser wavelengths (488 nm, 638 nm) and is also equipped with a system camera including Koehler lighting
Research into suitable fluorescent dyes and tumor-specific antibody stains was carried out by employees of the Fraunhofer IZI. After the first successful tests on fixed cells from cell cultures, a cooperation with the Helios Klinikum Erfurt was initiated, which provided tumor samples for testing the entire system after a positive ethics vote.
The entire LSC-Onco demonstrator consists of an optical scan head and a supply module (incl. laser and power supply). The mobile scan head with a total weight of < 5 kg is mounted on an automated x/y/z stage and can be positioned with respect to the sample using a joystick.
All work planned in the project for demonstrator development and in vitro testing on fixed human cells from cell culture and sections of patient material have been successfully completed.
The demonstrator LSC-Onco is available for further evaluation of tumor tissues (and other indications) at the Fraunhofer Center MEOS.
References
1. Falco, J. et al. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated With a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the FLUOCERTUM Prospective Study. Frontiers in surgery 6, 49; 10.3389/fsurg.2019.00049 (2019).
2. Bechtel, C., Knobbe, J., Grüger, H. & Lakner, H. Large field of view MEMS-based confocal laser scanning microscope for fluorescence imaging. Optik 125, 876–882; 10.1016/j.ijleo.2013.07.091 (2014).
3. Bechtel, C. Development of a MEMS-based confocal laser scanning microscope for fluorescence imaging. Zugl.: Dresden, Techn. Univ., Diss., 2014 (TUDpress Verl. der Wiss, Dresden, 2015).
4. Cavallo, C. et al. The utilization of fluorescein in brain tumor surgery: a systematic review. Journal of neurosurgical sciences 62, 690–703; 10.23736/S0390-5616.18.04480-6 (2018).
5. Yannuzzi, L. A. Indocyanine green angiography: a perspective on use in the clinical setting. American journal of ophthalmology 151, 745-751.e1; 10.1016/j.ajo.2011.01.043. (2011).
6. Porcu, E. P. et al. Indocyanine green delivery systems for tumour detection and treatments. Biotechnology advances 34, 768–789; 10.1016/j.biotechadv.2016.04.001. (2016).
 Fraunhofer Center for Microelectronic and Optical Systems for Biomedicine
Fraunhofer Center for Microelectronic and Optical Systems for Biomedicine
